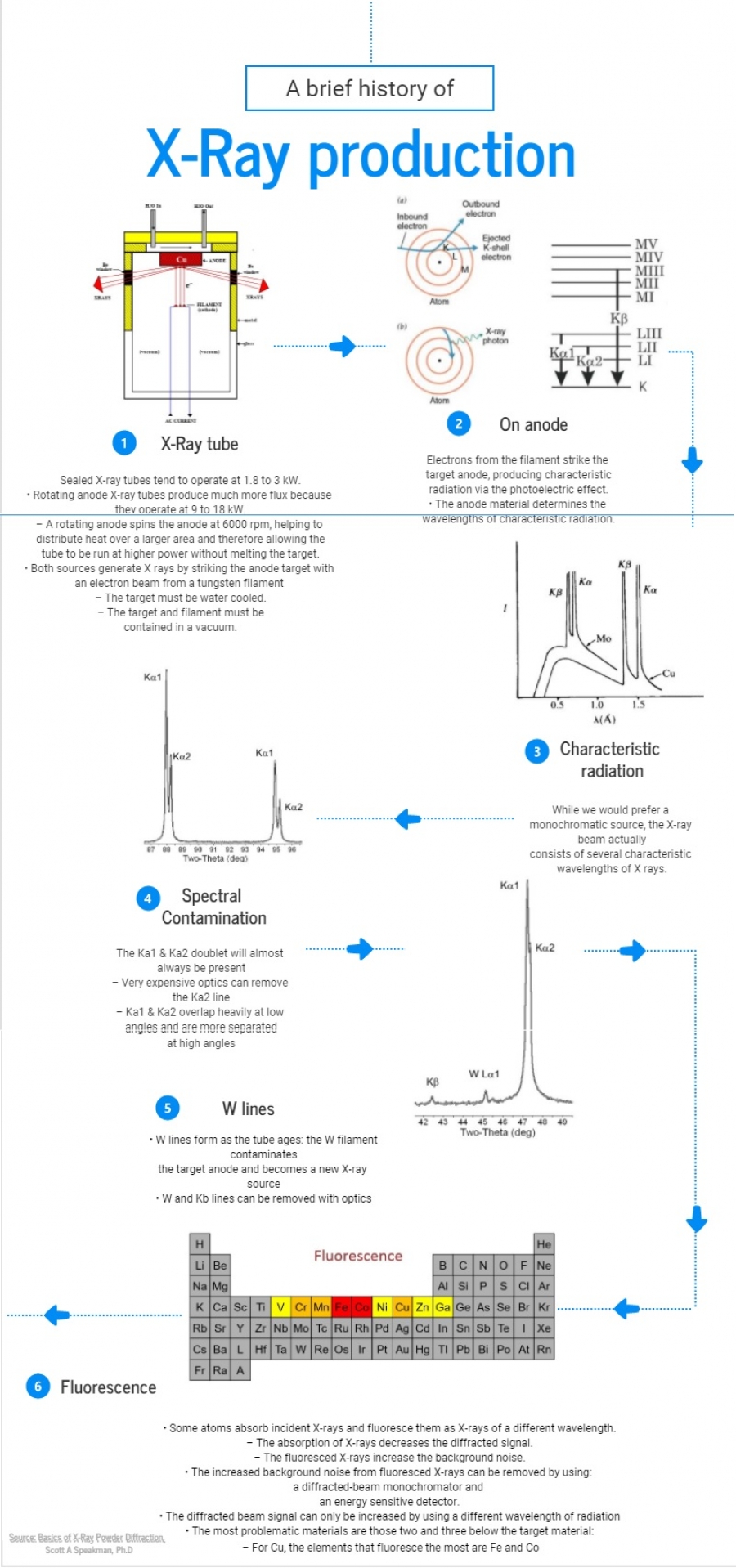
XRD is the experimental technique primarily used to determine the geometrical arrangement of the atoms or molecules within matter (crystal structure). When a crystal is bombarded by a beam of X-rays, X-rays are scattered by electron shells, and the angles through which the beam is diffracted reveal the shape and dimensions of the crystal’s unit cell. Diffraction experiments require the use of monochromatic Kα (or very close to monochromatic) radiation. This nearly monochromatic K α X-rays can be selected by the use of a filter or a monochromator. Since the wavelengths of Kα1 and Kα2 Xrays are so close in value, they are observed as a single Kα line when powder samples are probed. In XRD systems, single crystals are sometimes used as reference samples for calibration purposes. However, single crystals have narrow diffraction peaks that, when collected at high resolution, reveal doublets. To understand the origin of these doublets one needs to understand how X-rays are generated in a typical PXRD instrument. diffraction experiments. These characteristic X-rays are produced when high-energy electrons knock out the inner shell electrons of the anode material. The electrons from higher shells of the target atoms then drop down and fill the created vacancies and, in this process, emit X-rays with well-defined energy. For example, if a 1s electron from the K shell of copper atom is ejected, the resulting vacancy can be filled by an electron from the L shell (2p1/2 or 2p3/2) (I ask students why not from 2s orbital), the M shell (3p1/2 or 3p3/2), or the N shell (4p1/2 or 4p3/2). Subscripts 1/2 and 3/2 are the values of j, the total angular momentum quantum number.